Abstract
Through the NSF Future Manufacturing undergraduate research program at Pasadena City College (PCC), students utilize the tools of synthetic biology to build sustainable, DNA-based materials. The manipulation of DNA enables the construction of microscopic biochemical reactors through the formation of liquid-liquid phase-separated droplets, or DNA condensates. This research investigates the potential of DNA nanostars fused with G-tetraplexes, which can bind hemin, an iron-containing porphyrin co-factor, to form a DNAzyme capable of catalyzing peroxidation reactions within single condensate layers. The in vitro component of thisresearch was enhanced by in silico coarse-grained molecular dynamics simulations, which generated 3D models of the DNA nanostars that allowed student researchers to visualize the behavior of the structures created in the laboratory. Leveraging this computational technique, student researchers developed educational resources and modular lessons to introduce these molecular simulations to a broad student audience at PCC. The simulation programs used, oxDNA and oxView, were instrumental in making this research accessible and engaging for diverse student groups. DNA nanostar simulations were integrated into the General, Organic, and Biochemistry curriculum at PCC, as well as during outreach events such as Girls Science Day, offering students insights into DNA nanostar dynamics and potential applications of DNA-based inventions. This paper details the use of simulation programs to recreate nucleic acid-based nanostructures, advancing the field of DNA nanotechnology. Molecular simulations helped the PCC research students develop experiments that demonstrate how enzymatic activity within DNA droplets can be achieved through G4 complexing. Simulating DNA nanostars with G4s was a profound educational exercise for students, as it taught them about the powerful synergy between in silico and in vitro experimentation. Students also learned about the limitations of modeling biomolecules using computational software, and our G4 simulation results may even inspire the integration of guanine-guanine interactions into the oxDNA program. These findings underscore the significant implications of in silico modeling and structural analysis in biochemical manufacturing and industrial applications, paving the way for further innovations in programmable biomolecular systems. By developing YouTube tutorials that teach students how to carry out nucleic acid simulations on any standard computer, the exploration of DNA dynamics and molecular programming is now widely accessible to both students and educators.
Keywords: Undergraduate research; DNA nanotechnology; Synthetic Biology; Biomolecular condensates; DNA nanostar; DNAzyme; General Chemistry, Organic Chemistry; Biochemistry; Molecular Dynamics Simulations; scientific method; workforce development; STEM education and outreach; equity in science and engineering; Women in STEM
© 2025 under the terms of the J ATE Open Access Publishing Agreement
Introduction
Due to the predictability, stability, and programmability of Watson-Crick-Franklin base pairing, nucleic acids have emerged as an excellent material for building microscopic structures with programmable and controlled interaction capabilities [1, 2]. One such structure, known as a DNA nanostar, consists of three or more DNA strands that assemble through complementary base pairing, with each strand carefully designed to ensure proper binding. In this research, four-stranded DNA nanostars were used. The structure of the DNA nanostar comprises three main components: the core sequences, the sticky ends, and the spacer regions (Fig. 1). The core sequences (CS) are composed of nucleotides designed to complement and adhere to one another, forming the main body of the nanostar and giving rise to its double-stranded “arms.” The spacers (SP), non-palindromic and located at the center of the nanostar, provide flexibility to the arms. The sticky ends (SE) are present on all core strands at the terminus of the arms, facilitating intermolecular interactions and self-assembly of the different nanostars [2].
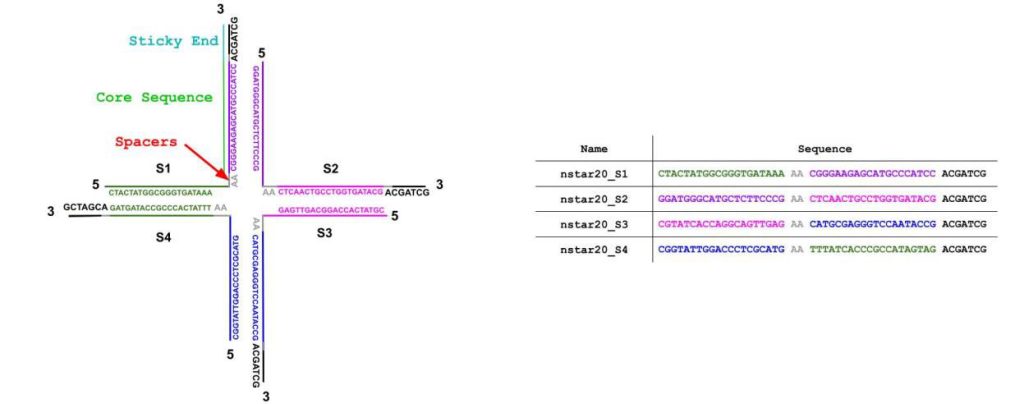
In this study, the four-armed DNA nanostar structure was modified by extending one of the core sequences (CS) on strand four to include a DNAzyme-encoding sequence. While DNAzymes are a diverse class of catalytic DNA molecules, certain guanine-rich DNAzymes rely on G-quadruplex formation to interact with hemin (a cofactor) and catalyze peroxidation reactions. The added sequence folds into a G-tetraplex (G4), which subsequently binds hemin, forming a catalytically active complex that exhibits peroxidase activity. This DNAzyme complex facilitates peroxidation reactions, such as those between hydrogen peroxide and ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)), a colorimetric reducing agent (Fig. S1). Characterizing the folding and catalytic efficiencies of DNAzymes under various conditions is crucial, given their immense potential applications in diagnostics, biosensors, and other areas of bionanotechnology [4]. By simulating the folding of the G-quadruplex complex with the DNA nanostar, student researchers could visualize the dynamic structure, enabling deeper understanding and the development of innovative approaches to programming DNAzyme-catalyzed reactions.
DNA nanostars interact to form a network, which leads to condensation into phase-separated compartments. Synthetic DNA condensates provide a compelling model of membraneless organelles, components of living cells that have recently emerged as a significant area of study in biological research. Understanding the properties and functions of membraneless organelles may provide insight into the origins of life and enable engineering of biomolecular condensates that localize reagents and reactions in (artificial) cells by design, with potential applications in the fields of synthetic biology, nanomedicine, and biopharmaceuticals [3]. Given the incredible promise of DNA in bionanotechnology, models that elucidate the structural dynamics of DNA assemblies are invaluable for interpreting experimental data and informing future synthetic DNA designs. To this end, oxDNA [5], a coarse-grained molecular dynamics software developed by researchers at the University of Oxford [6], and its companion program, oxView, were used to investigate the structural and mechanical properties of DNA nanostructures. These tools were central to the objectives of this study. OxDNA builds on the coarse-grained DNA model established by T.E. Ouldridge, J.P.K. Doye, and A.A. Louis [6–10], providing a robust framework for simulating DNA behavior. OxView complements oxDNA by enabling the creation of job codes and structural models for input on simulators like oxDNA [6-10]. Together, these programs offer an accessible and accurate means of modeling DNA nanostructures, such as the DNA nanostars featured in this research. Simulations were carried out on nanostars with 15, 20, and 25 base pairs per arm, with and without guanine-rich DNAzyme extensions, to explore how liquid-liquid phase separation (LLPS)—the spontaneous separation and formation of molecular aggregates into two distinct phases—might occur based on arm length and sticky end sequence [3, 11]. The simulations also examined how the addition of a DNAzyme sequence to a DNA nanostar could promote the formation of a peroxidase-like quadruplex structure [4, 11].
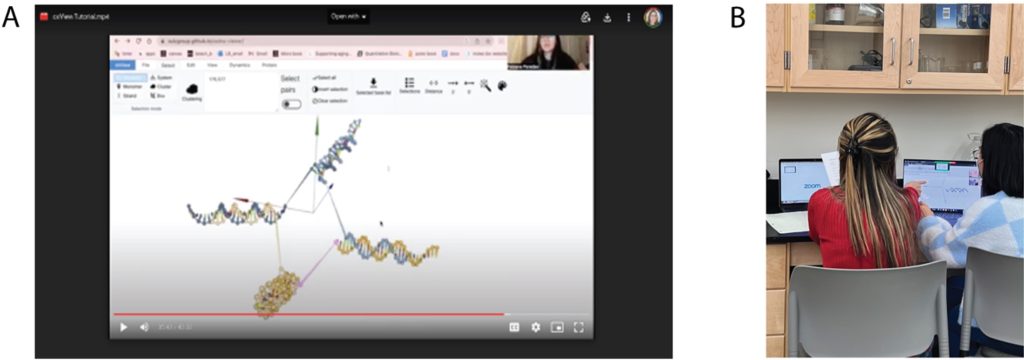
Through the NSF Future Manufacturing research program at Pasadena City College (PCC) in collaboration with UCLA, UCSB, and Caltech, a team of student researchers—referred to as the ‘PCC nanostars’— employed the tools of synthetic biology to localize biochemical reactions within DNA condensates. The PCC nanostars began their training at a research techniques bootcamp hosted by the Franco laboratory in the Bioengineering Department at UCLA, where they learned relevant methods and strategies in the field of DNA nanotechnology from experts in the field. Graduate students, postdocs, and professors guided the PCC students through hands-on training sessions and lectures regarding theory and current research in their laboratories, which focuses on nucleic acid condensates. The computational research presented in this study was initiated when a graduate student from Prof. Franco’s laboratory taught the PCC nanostar team how to design DNA nanostructures using oxView and simulate their dynamics in oxDNA (Fig. 2). Building on these foundational skills, the PCC researchers conducted in silico experiments to simulate the DNA nanostars they had designed for in vitro experiments. Student-driven experimentation led to simulations of G4 structures LA4 and 5xG3T (Table 1) extended from arm 4 of the NS-15, NS-20, and NS-25 DNA nanostars. The researchers varied simulation parameters to visualize the folding of these G4 structures and gain insight into their dynamic behavior. Simulating DNA nanostars with G4s was a profound educational exercise for students, as they experienced the synergy between in silico and in vitro experimentation. Students also learned about the limitations of modeling biomolecules using computational software. With the aim of translating this synthetic biology research into accessible educational content, the PCC nanostar team created YouTube tutorials to teach students how to simulate DNA nanostars using oxView/oxDNA [12]. These tutorials were incorporated into Prof. Jillian L. Blatti (Dr. JB)’s General, Organic, and Biochemistry (Chemistry 2B) courses at PCC during the Spring 2023–2025 semesters as part of a new curriculum focused on the PCC nanostars’ synthetic biology research. The lessons and resources were also used in STEM outreach events, such as Girls Science Day (Fig. 3), making cutting-edge research accessible to a diverse student audience. As students engaged in hands-on activities, forming in vitro DNA nanostar condensates and visualizing them through fluorescence microscopy, their experiments were complemented by in silico simulations using oxDNA, allowing them to model, simulate, and analyze the mechanical properties, hybridization dynamics, and interactions of the DNA nanostars. By observing the kinetics of nucleic acid nanostructures and visualizing dynamic molecular events that are difficult to detect experimentally, students gained a deeper understanding of the connection between life science theory and real-world molecular behavior.
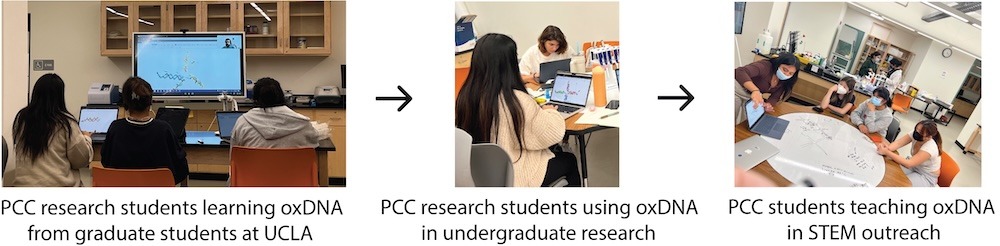
Accessible STEM education is a significant aim of the NSF Future Manufacturing research program. By creating YouTube tutorials that teach students how to design, build, model, and form DNA nanostructures, we have reached a broad and diverse audience. oxDNA simulations played a significant role in STEM outreach efforts, particularly during Girls Science Day, when visiting students (ages 10–14) had the opportunity to create and visualize DNA nanostructures through microscopy. Though they were amazed by the ‘glowing DNA liquids’, it was the dynamic DNA nanostar simulations that truly captivated them, showcasing the ‘dancing DNA nanostars’ and their dynamic movements. These nucleic acid simulations allowed students to connect abstract molecular concepts to tangible, visual representations. This hands-on scientific experience fostered a deeper appreciation for the nanoscale world, making complex science more accessible to students and inspiring their enthusiasm for molecular biology and biochemistry. Furthermore, the PCC nanostar researchers served as role models to the young students as they facilitated the DNA nanotechnology lessons; their enthusiasm for the research was evident and infectious to the young girls as they taught them how to use synthetic biology to imagine, design, and build functional nanostructures. Notably, YouTube tutorials on how to design nanostructures using DNA origami (published in March 2020) have views in the order of thousands with hundreds of hours of watch time; and although published recently (August 2024), the DNA nanostar simulation tutorials already have upwards of 400 views [12].
Methods
Forming DNA Nanostars
A series of fluorescent DNA nanostars was designed, all with four arms and either 15-, 20-, or 25-base pairs in each arm, a center composed of 2 adenine bases per strand, and 6 base-pair sticky ends (Fig. 1, Tables 1-3). To facilitate condensate visualization, 5% of the DNA nanostars contained a fluorescent molecule (e.g., Yakima Yellow) attached to arm 1 (Fig. 4). 50% of the designed nanostars incorporated a G-tetraplex sequence extended from arm 4 (Tables 1-3). These DNA strands were purchased from Integrated DNA Technologies (IDT), then quantified via absorbance measurements from a Nanodrop One UV-Vis Spectrophotometer at 260 nm. Each strand (except for the strand 1 with fluorescent dye attached and the strand 4 with the G4 attached) was diluted tenfold prior to quantification, and the actual concentrations from IDT were backcalculated using Beer’s Law and the extinction coefficients provided on the oligonucleotide specification sheets.
Table 1. DNA sequences for DNA nanostar 15 (NS-15) formation with and without a G4
| Name | Sequence |
| nstar15_strand1C | CTAGTCTACAGTGCC AA CTGGGCAGAATTCCC AGCTAGC |
| nstar15_strand2 | GGGAATTCTGCCCAG AA CGTCACCAGAAGCAC AGCTAGC |
| nstar15_strand3 | GTGCTTCTGGTGACG AA GACGGAATCTCCGTC AGCTAGC |
| nstar15_strand4 | GACGGAGATTCCGTC AA GGCACTGTAGACTAG AGCTAGC |
| nstar15_strand4G-LA4 | GGGTGGGAAAAGGGTGGG GACGGAGATTCCGTC AA GGCACTGTAGACTAG AGCTAGC |
| nstar15_strand4G-5xG3T | GGGTGGGTGGGTGGGTGGGT GACGGAGATTCCGTC AA GGCACTGTAGACTAG AGCTAGC |
Table 2 DNA sequences for DNA nanostar 20 (NS-20) formation with and without a G4
| Name | Sequence |
| nstar20_strand1 | CTACTATGGCGGGTGATAAA AA CGGGAAGAGCATGCCCATCC ACGATCG |
| nstar20_strand2 | GGATGGGCATGCTCTTCCCG AA CTCAACTGCCTGGTGATACG ACGATCG |
| nstar20_strand3 | CGTATCACCAGGCAGTTGAG AA CATGCGAGGGTCCAATACCG ACGATCG |
| nstar20_strand4 | CGGTATTGGACCCTCGCATG AA TTTATCACCCGCCATAGTAG ACGATCG |
| nstar20_strand4-LA4 | GGGTGGGAAAAGGGTGGG CGGTATTGGACCCTCGCATG AA TTTATCACCCGCCATAGTAG ACGATCG |
| nstar20_strand4-5xG3T | GGGTGGGTGGGTGGGTGGGT CGGTATTGGACCCTCGCATG AA TTTATCACCCGCCATAGTAG ACGATCG |
Table 3. DNA sequences for DNA nanostar 25 (NS-25) formation with and without a G4
| Name | Sequence |
| nstar25_strand1 | CGCTACAATACAGTTACAAGAATGC AA CGCTTGATGTATGCACGTATGTTGC ACACGTG |
| nstar25_strand2 | GCAACATACGTGCATACATCAAGCG AA CATATCTCATATTCGTGCCACTATG ACACGTG |
| nstar25_strand3 | CATAGTGGCACGAATATGAGATATG AA CAGTAGGGCAGCAAAGACTACGGTG ACACGTG |
| nstar25_strand4 | CACCGTAGTCTTTGCTGCCCTACTG AA GCATTCTTGTAACTGTATTGTAGCG ACACGTG |
| nstar25_strand4-LA4 | GGGTGGGAAAAGGGTGGG CACCGTAGTCTTTGCTGCCCTACTG AA GCATTCTTGTAACTGTATTGTAGCG ACACGTG |
| nstar25_strand4-5xG3T | GGGTGGGTGGGTGGGTGGGT CACCGTAGTCTTTGCTGCCCTACTG AA GCATTCTTGTAACTGTATTGTAGCG ACACGTG |
To form the microscale condensates, tenfold-diluted strands of the DNA nanostar were added to the reaction tube to achieve a final concentration of 20 µM, with the exception of strand 1 in which 5% contains a fluorescent dye attached. The strand 1 with fluorescent dye was diluted to a concentration of 50 µM prior to adding it to the reaction tube. For the condensates that possess a G4 on strand 4, 50% of strand 4 extends the sequence for the G4 (10 µM final concentration of each strand type). Additionally, a final concentration of 350 mM KCl (purchased from Thermo Fisher Scientific) and 20 mM Tris-HCl, pH 7.0-7.5 (purchased from Sigma-Aldrich) are required to form the catalytic DNA condensates as the potassium ion increases the catalytic efficiency of the DNAzyme [13, 14] (Table S1).
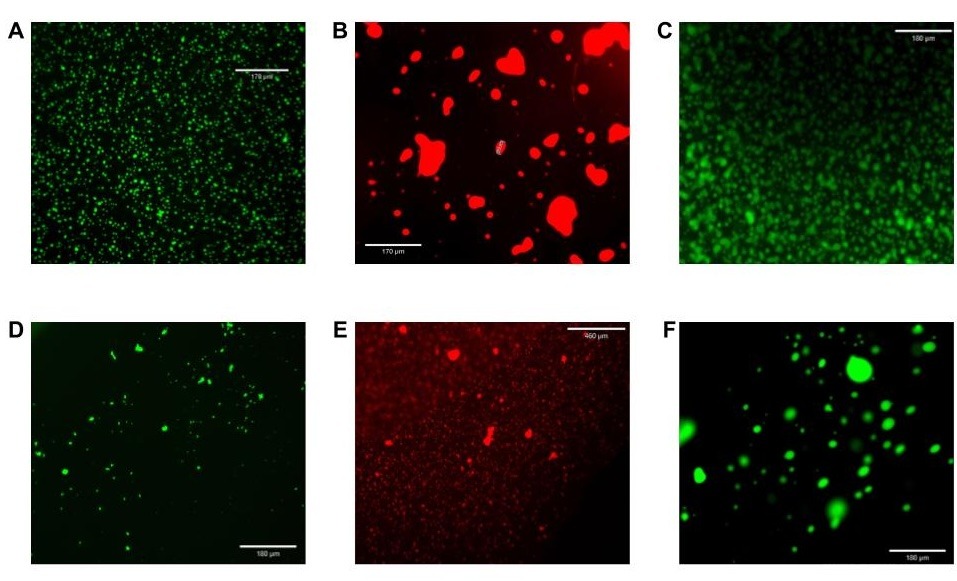
In the laboratory, DNA nanostars were annealed in vitro by placing the solutions in a thermocycler set to 95°C for ten minutes, then slowly cooling to room temperature at a rate of 1°C per minute. The corresponding DNA condensates were imaged by adding 2.5 µL of this mixture into the center of a Parafilm chamber on a microscope slide, placing a cover slip upon the chamber with DNA condensate solution, and viewing under an Echo Revolve Fluorescence Microscope. Fig. 4 depicts fluorescence micrographs of NS15/FAM, NS-20/Texas Red, NS-25/Yakima Yellow, NS-15/FAM/50%LA4, NS-20/Texas Red/50%LA4, and NS-25/Yakima Yellow/50%LA4.
Simulating DNA nanostars using oxView
To computationally model and visualize the dynamics of DNA nanostars, oxView was used to construct the nanostructures with and without G4/DNAzyme sequences in silico, which were then simulated and analyzed using oxDNA. Simulating a DNA nanostar without a G-tetraplex involves understanding its three main components: sticky ends (SE), core sequences (CS), and spacers (SP) (Fig. 1). This detailed, multistep procedure ensures the nanostar’s accurate construction, precise definition of its structural components, and preparation for simulation. Given the complexities of designing nanostructures computationally— requiring exact sequence input and alignment—this paper provides step-by-step instructions to help users accurately replicate the process (see Supporting Information pp. 2-15) along with DNA sequences for input (Table 1-3). The oxView software plays a crucial role, generating two critical files (.dat and .top), which serve as inputs for oxDNA to analyze the nanostar’s dynamic behavior. To extend and form a G-tetraplex on arm 4 of the nanostars, additional steps are required and described. Two different DNAzymes were selected, studied, and compared to simulate the folding of the G-tetraplex: LA4 and 5xG3T (Table 4).
Table 4. DNAzyme sequences used in simulations
| Name | Sequence |
| LA4 | GGGTGGGAAAAGGGTGGG |
| 5xG3T | GGGTGGGTGGGTGGGTGGGT |
The oxDNA simulations of DNA nanostars (e.g., Fig. 5) were turned into GIF movies that illustrated the dynamic movements of the nanostructures [15]. This was very engaging to students and worked in synergy with the in vitro formation of DNA nanostar condensates in the laboratory, facilitating an important conceptual understanding of these dynamic nanostructures for students. The simulation captured the interactions and spatial organization of individual nucleotides, effectively mirroring the real-life structural behavior of the DNA nanostar. This molecular simulation brings the conceptual nanotechnology to life, and students reported that it “helped reinforce my understanding of how DNA and enzymes interact at the atomic level.”
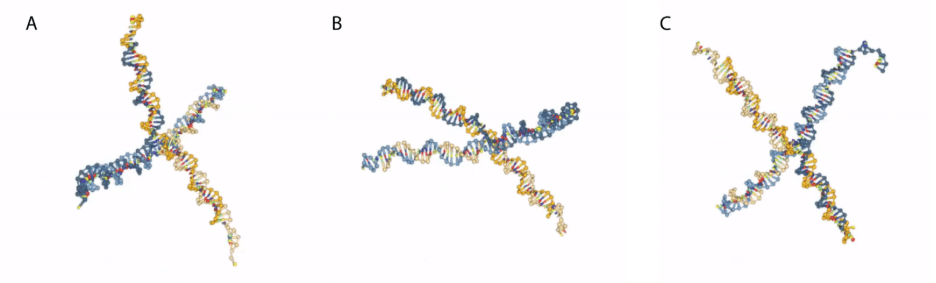
Fig. 5. Snapshots of dynamic simulation of 4-armed nanostars created on oxView and simulated using oxDNA. See reference [15] for the dynamic movie of the DNA nanostar simulation.
Results and Discussion
oxDNA simulation of 15, 20, 25 BP/arm, 4-arm DNA nanostars with and without G-tetraplexes
After creating and running the oxDNA simulations in accordance with the steps provided in the SI, with the default settings of 109steps (determines the length of the simulation run) at 1 M salt concentration, nanostars with 15, 20, and 25 base pairs per arm were successfully created (Fig. 6). Given the larger size of nanostar 25, its structure in the simulation appears to be the least flexible out of the three nanostars, suggesting it is the most stable.
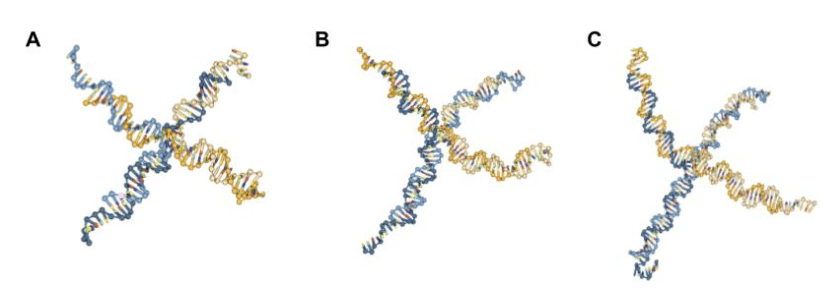
Fig. 6. Snapshots of oxDNA simulation of 4-arm DNA nanostar [6-10], with: (A) 15 base pairs per arm; (B) 20 base pairs per arm, and (C) 25 base pairs per arm.
After creating and running the oxDNA simulations in accordance with the steps provided regarding the addition of the DNAzyme sequence (SI) with the default settings of 109 steps at 1 M salt concentration, the results when attempting to fold the G4 quadruplex were less clear (Figs. 7, 8). Despite its reputation for stability under extreme conditions [4, 16], in silico simulations of the folding of the guanine-rich DNAzyme yielded instability. The DNA nanostars with the folded G4 sequences presented in Figures 7 and 8 are screenshots taken during times in the simulation when the G4 complex structure was apparent. However, throughout the time of the simulation, this G4 sequence was constantly folding and unfolding. Other sources, including those written by some of the oxDNA program creators themselves, report similar issues with G-quadruplex folding, noting that the oxDNA program does not currently support noncanonical base pairs [17, 18-19]. Despite this, the process of creating and simulating a nanostar with a G4 sequence can still provide some insight into how the quadruplex would look in vitro at times when the sequence is folded in oxView.
To understand the structures they were forming in vitro, the PCC nanostars designed in silico experiments in which the 4-arm, 15, 20, and 25 base pairs per arm DNA nanostars were simulated with two different G4 sequences–LA4 and 5xG3T (Table 1). Both DNAzymes showed folding into the G4 quadruplex structure at some point during the simulation and the same instability of the folded structure (Figs. 7, 8). A key difference, however, was that 5xG3T was observed to fold less often and/or to a lesser extent into a quadruplex structure in comparison to LA4.
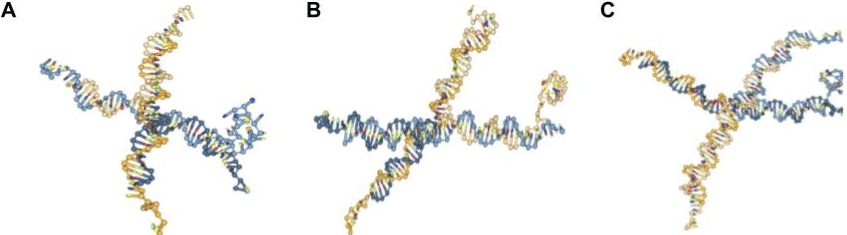
Fig. 7. Screenshots of oxDNA simulation of 4-arm DNA nanostar [6-10], with: (A) 15 base pairs per arm and G4 quadruplex LA4 extended from arm 4; (B) 20 base pairs per arm and G4 quadruplex LA4 extended from arm 4; (C) 25 base pairs per arm and G4 quadruplex LA4 extended from arm 4.
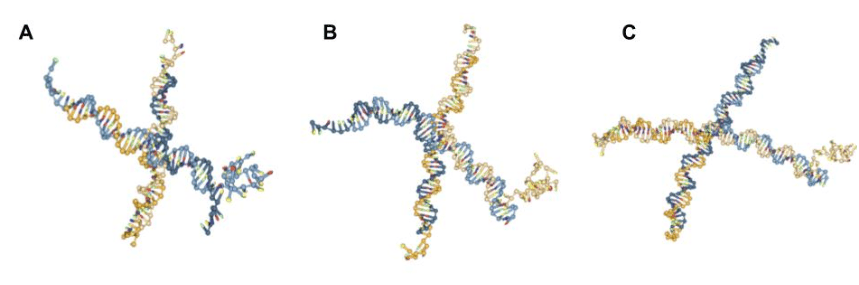
Fig. 8. Screenshots of oxDNA simulation of 4-arm DNA nanostar [6-10], with: (A) 15 base pairs per arm and G4 quadruplex 5xG3T extended from arm 4; (B) 20 base pairs per arm and G4 quadruplex 5xG3T extended from arm 4; (C) 25 base pairs per arm and G4 quadruplex 5xG3T extended from arm 4.
Simulating catalytic DNA nanostars with G-tetraplexes extended from arm 4: Varying the experimental conditions in oxDNA to attempt to fold the G4
Before achieving the folded G-quadruplexes seen in Figures 7 and 8, several experiments were conducted on nanostar 15 with LA4 to determine the best conditions to attempt to fold the DNAzyme. First, the folding of the DNAzyme was attempted without the use of an external force file. Changes were made to the temperature, number of steps, salt concentration, and simulation time step (dt), etc. [5, 20] to observe whether any impact was made on the folding of the DNAzyme (Fig. 9A-D).
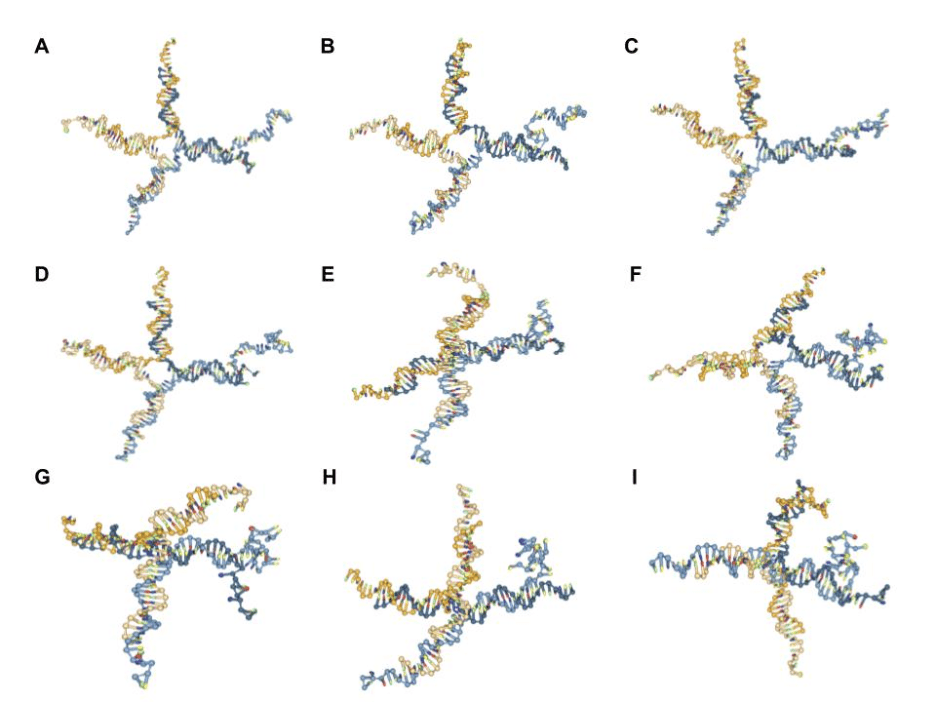
Fig. 9. Screenshots of testing various conditions to fold G4 quadruplex [6-10], with: (A) oxDNA default settings; (B) 1020 additional steps; (C) 1020 additional steps and 1.5 M salt concentration;
(D) 1020 additional steps and 0.5 M salt concentration; (E) external file with default stiffness of 0.09; (F) external file with stiffness of 6.09; (G) external file with stiffness of 8.09 and 109 additional steps; (H) external file with stiffness of 8.09 at 35 ℃; (I) external file with stiffness of 8.09 and dt = 0.005.
By simulating DNA nanostar 15 with LA4 attached to arm 4 with a few orders of magnitude greater number of steps (Fig. 9B), a small improvement was observed in terms of folding the G4. Based on this finding, several additional changes were made to the nanostar achieved in Figure 9B, including attempts to change the temperature (ranging from 16 ℃ to 60 ℃) and time step (increase to 0.005 from 0.001) of the simulation, which had little to no effect on folding. In contrast, both increasing (1.5 M) and decreasing (0.5 M) the salt concentration of the simulation were observed to have a small negative effect on the folding of the G4 (Fig. 9C-D). The main conclusion from these sets of experiments was that none of the adjustments to the oxDNA simulation settings made a marked impact on creating the desired G4 quadruplex structure, and similar results have been observed by others attempting to fold G quadruplexes [17-19].
After determining that none of these methods were sufficient in folding the DNAzyme, it was discovered that the use of an external force file would be necessary to attempt to accelerate the formation of the secondary G-quadruplex DNA structure [16, 20-21]. From there, oxView’s force implementation setting was realized, and the positive impact of an external file was noted. Then, the stiffness of the forces on oxView was increased [17, 21], and its effect on the folding of the DNAzyme was observed (Fig. 9E-I). Although a stiffness of 6.09 for forces on oxView had similar results to the 8.09 value utilized in this paper, the higher value was chosen due to a slight positive effect. Furthermore, adding additional steps, implementing a higher temperature, or a slightly higher time step (0.005 instead of 0.001) did not make a significant difference on folding (Fig. 9G-I).
oxDNA simulation of DNA nanostars through undergraduate research at PCC
oxView and oxDNA were invaluable to the research conducted through the NSF Future Manufacturing undergraduate research program at PCC. As previously mentioned, the PCC nanostars participated in a research techniques bootcamp at UCLA in the Franco Lab, where PCC researchers collaborated with and learned from experts in the field of DNA nanotechnology. Following the bootcamp, they began authentic research at PCC, where the research goal is to localize DNAzyme-catalyzed peroxidation to DNA condensates. Toward this aim, the first step was to form and visualize DNA nanostars with and without DNAzymes extended from arm 4, while optimizing DNAzyme-catalyzed peroxidation [14]. DNA nanostars were designed to have four arms, and either 15, 20, or 25 base pairs per arm and a fluorophore attached to arm 1 for visualization (Fig. 1). To achieve localization of DNAzyme-catalyzed peroxidation, arm 4 in each of these systems was extended with the sequence for the G4 quadruplex, which could fold, bind hemin and become a functional DNAzyme complexed with a DNA nanostar. To better understand and visualize the nanostructures—something unattainable without molecular modeling and simulation— student researchers learned to use oxDNA and oxView. The goal quickly extended beyond visualization; inspired by biomimicry, the PCC nanostars attempted to simulate the chain-like interactions between different nanostar droplets. The researchers first tested whether colorimetric peroxidation of ABTS could occur on a standalone G4 and also on a G4 integrated into one of the nanostar arms [14, 22]. Through the simulation of the three different DNA nanostars available in the laboratory—with 15, 20, or 25 base pairs per arm with and without G4—properties detected in in vitro experiments were also observed in silico.
Reflections from the PCC researchers: using oxDNA to simulate DNA nanostars and its impact on research and understanding of the DNA nanostructures
To get a sense of the impact of the use of oxDNA and simulations in research and outreach, surveys were given to the PCC nanostar researchers. The words from the PCC nanostar researchers follow below.
“I gained a lot of satisfaction once I could see a visualization of the structure of the DNA nanostar because it’s different from just looking at them in the form of a DNA condensate through a microscope or in a PCR tube. These tools made me realize that the use of modeling and simulation can be necessary for scientific research, even if it’s just for visualization purposes.” Gracious
“When we’re working with and learning about reactions at the microscale, it can be hard to grasp what’s truly happening since the DNA structures cannot be seen by the naked eye. Becoming familiar with oxDNA and oxView has transformed my understanding of our research in DNA nanotechnology and my understanding of DNA as a programmable material. This experience has taught me about the importance of modeling in scientific research, and I hope to see similar simulations implemented in classrooms.” Megan
“Visualization is one of the hardest parts of working with nanoscale structures. To make progress in any bioengineering research, there needs to be a strong understanding of what is happening at the nanoscale, and modeling and simulations help with this. Using oxDNA put an image in my mind of what a DNA nanostar looks like and how its complementary pieces come together to form the final structure. Modeling is a valuable tool for visual learners and can enhance a deeper understanding of complex ideas. I experienced this firsthand and hope to see more of it used in all areas and levels of education.” Cynthia
“Creating DNA nanostars demands a high level of precision and meticulous attention to detail, making it an essential foundation for microscale research. The experience of forming enzymes from DNA is not only gratifying but also prompts an intriguing question: what do our creations actually look like? Employing oxDNA to simulate our nanostars filled this gap. This powerful integration of computational tools effectively connects hands-on experimentation with tangible results, enriching our understanding of DNA nanostructures. My hope is that by showcasing this approach, we can motivate students to merge experimental work with simulation, elevating their research outcomes and overall learning experience.” Fabiana
“Throughout our research process, most of it involved experiments visualized on the macroscale and microscale. Because the nanostar droplets are the only indication of the nanostars forming, the utilization of oxDNA provided me with much more clarification on their structures and how they function and form. It was also fascinating to see how the DNAzyme’s sequence attaches to the DNA nanostar, which is ultimately what allows the peroxidation reaction to happen and become localized. Seeing this form of computational modeling and simulation allowed me to see its vast range of applications in different aspects of biotechnology research and how far experimental techniques have come.” Eliana
“As I progressed through the program, I was able to develop a more in-depth understanding of nanoscale reactions and structures, with oxDNA being the driving force. Initially, I understood the basic functions of the reagents and the product of the experiments on a large scale. I focused on the colorimetric aspect and what this meant for the solution as a whole. As the program went on, we advanced to microscopy, and we were able to see this larger solution broken down into its smaller droplets. Then, our oxDNA work provided clarity on every level of the project. We were able to understand the morphology of these nanostars and the significance down to the nucleotide sequence. This helped solidify our team’s purpose while providing us with the skills to become more efficient researchers.” Britney
“The integration of oxDNA into our research contributed more than just technical skill development and experience with computer simulation; it creatively translated an abstract idea to its real, physical form. The opportunity to construct the nanostar from the “ground up” using technological tools meant truly comprehending the scientific “magic” that existed in the PCR tubes of nanostar solutions we worked with and comprehending their potential to catalyze reactions and form programmable biocompartments. This depth of learning is invaluable and allows students, researchers or otherwise, to personally connect to the science being witnessed. Understanding the complexity of forces at work in a nanostar network still inspires me to pursue research and think at the nanoscale, even today as part of another nanoengineering-based lab!” Reina
“At first, oxDNA was a fascinating and exciting introduction to scientific modeling that helped me visualize the focus of our in vitro experiments–DNA nanostars–in a way that was not possible to see using our microscopy techniques. However, as my simulation attempts transitioned from forming DNA nanostars without G4s to experimenting with ways to try to get a G4 on the nanostar to fold, I encountered frustrations and setbacks. Despite these challenges, this process taught me a valuable lesson about the scientific method–to face failure head-on and to reimagine and rethink my approach continuously. These in silico experiments truly impressed upon me a deep appreciation for the research process and have inspired me to pursue research in the future.” Emily
“Exploring oxDNA during the program has greatly improved my understanding of the molecular features of DNA nanostructures. Using oxDNA allowed me to obtain a better knowledge of the interactions and dynamics of the strands I was dealing with, both chemically and physically. This computational tool helped me bridge the gap between theoretical understanding and practical applications, allowing me to visualize complex molecular dynamics and improve my nanoscale data interpretation technique. The insights supplied by oxDNA were critical in moving our research forward, providing me with the analytical viewpoint and abilities required to make a substantial contribution to the field of molecular biotechnology.” Taneeka
“Working with oxDNA was the first time I have ever modeled 3D structures. The end product visualized through oxView allowed me to better grasp the DNA nanostar structure. Rather than the flat drawing I’m used to seeing when planning the next project, the simulation allowed me to better visualize the steric allowances and predicted behavior. The technological side of science was something I was not familiar with prior to working with oxDNA. Having the opportunity to learn more about this program and apply it to actual research has helped bridge the initial hesitancy I had about traversing down this field. I think that starting off computational research through programs like this, in and outside of a classroom setting, can greatly benefit individuals who might want to explore this area of science.” Wing
“Prior to plotting out the nanostar strands on oxDNA, my understanding of the structure was very elementary. Physically linking the base pairs on this program highlighted for me just how calculated the sequences are. Moreover, I was in awe of the potential and endless possibilities granted by methodical nucleic acid design. Once I had a more dynamic portrait of our nanostar structures’ essence, I was able to conceptualize their capacity to hold not just any additional appendages but specific functional DNAzymes, responsive to stimuli– all with a purpose. I mean, genuinely, each piece of this scientific investigation was of intentional design, something that I couldn’t have ever dreamed up without this hands-on experience.” Vanessa
Molecular simulations of DNA nanostars in teaching and outreach
With guidance from research advisors, PCC nanostars designed and implemented lessons to teach concepts and techniques they developed through their undergraduate research in DNA nanotechnology. Over the course of two months of research, experiments were optimized and then translated into a series of lessons, advancing both the science and the students’ ability to communicate complex ideas effectively. This research program offers the opportunity for student researchers to practice communicating their science, develop lesson plans based on their research, and then implement the lessons in a classroom setting— a unique experience that inspires everyone involved. Mentoring research students in equitable teaching and outreach has become a hallmark of this research program, and it has identified talent in scientific teaching. As the PCC nanostar student researchers taught peers and younger students about the science they developed, they offered their expert advice, support, encouragement, and enthusiasm for the science. When students saw, for the first time, the fluorescent DNA nanostar condensates on the Echo Revolve microscope, they were amazed! Further, when they observed their dynamic DNA nanostar simulations, they felt incredibly proud of their in silico work. PCC nanostar researchers served as inspirational teachers, guiding students as they created and viewed simulations of DNA nanostars, which gave them deep insight into the structures they were making and visualizing in the laboratory (Figs. 2,3).
In the Spring 2023 – 2025 semesters at PCC, General, Organic and Biochemistry (Chemistry 2B) students engaged with a new curriculum centered on the synthetic biology research conducted by the PCC nanostar student researchers. As part of this new curriculum, students annealed DNA nanostars in vitro and visualized the resultant DNA condensates through microscopy, and they simultaneously used oxView and oxDNA to simulate them. The PCC nanostars served as learning assistants, guiding the Chemistry 2B students through these activities. During the first week, students were given instructions to carry out an oxDNA simulation of either NS15, NS20, NS25, which included a guided worksheet (SI) and YouTube tutorial [12]. The 24-student class was split into six groups of four, and during the first week of the 5week DNA nanotechnology curriculum, half of the class conducted simulations, and the other half conducted a laboratory activity in which they formed fluorescent DNA nanostar condensates and visualized them on an Echo Revolve fluorescence microscope. The DNA nanostars that the students simulated in silico were the same DNA nanostars that they formed in vitro; for example, students who simulated NS-15 using oxDNA also formed and visualized NS-15 condensates in the laboratory. Chemistry 2B students were engaged in the simulations and visualizing the fluorescent DNA nanostar condensates, which they reported on post-laboratory assessment surveys. Some students commented that the oxDNA simulations were challenging but rewarding; most students enjoyed the activity. The PCC student researchers felt very accomplished as science educators, and they had fun creating the lessons and teaching them in a collegiate setting.
“Engaging with students as an instructional assistant and actively contributing to outreach workshops proved to be a very wholesome and uniquely rewarding experience. Not only did these opportunities lead to and contribute to lessons that strengthened my understanding of the research, but they deepened my passion for serving the students, as well as for the science that constituted the study of these miraculous nanostars. Using oxDNA as an instructional tool allowed for an accessible research communication and learning tool to be used with the goal of inspiring budding scientists, especially from marginalized demographics. I can confidently state that being so intimately involved in this mission has changed my perspective on equity efforts and academia forever.” Reina
“Our research project, including the protocols and results adapted for this manuscript, was part of a broader effort to make research more accessible and inclusive for community college students. The dedication of our principal investigator, Dr. Jillian Blatti, and collaborators at UCLA, Caltech, and UCSB to foster equity in research opportunities was both inspiring and transformative. The teaching and outreach opportunities I participated in during this project solidified my commitment to science as an aspiring educator. Facilitating a lesson on DNA nanostars in a chemistry class and inspiring middle school girls to explore STEM were made more impactful by the ability to visualize our research outcomes through oxDNA. This powerful tool not only showcased the beauty of our work but also fostered enthusiasm and accessibility for those new to scientific research.” Fabiana
“So it turns out that being meticulous scientists with attention to detail pays off. Our successes from our time as researchers were able to be replicated and shared with our Pasadena City College community. Sorting through various instrument manuals, fine-tuning protocols, and dissecting our microscopic visualizations were all critical in our familiarization process. To even get to the point of being able to present our results to each other and even draw from literature, which also required understanding, was a feat we were able to achieve with Dr. Jillian Blatti’s guidance and diligence. Our cohorts have exponentially grown in their familiarization with DNA nanotechnology, canonizing the oxDNA mapping, which served as a strong foundation for further investigation. I am excited at the possibilities that DNA nanotechnology holds and the potential for fostering dynamic learning environments, and especially excited to make cutting-edge science accessible to all curious minds.” Vanessa
“The student interactions were very positive! It was so fun and I never felt any sort of authority within our learning environment. It certainly made it easier to share knowledge between students and facilitators. My favorite thing was learning from students. They taught me hard work and respect for professors like Dr. J.B., who put so much time towards making the material more engaging and accessible to us. My favorite thing was seeing them so engaged that they began to form questions and hypotheses!” Britney
“I really enjoyed how excited everyone was to see their results. When the slides are put underneath the fluorescence microscope, and the images pop to life, the joy present is something that I feel lucky to have been able to witness. Even during the microplastic experiment, I remember the tenacity and preservation from the students when obstacles arose. I still remember how a group of students stepped forward to solve an issue when the filter did not fit the water sprout. They worked hard to make things work and came together as a class to solve the problems.” Wing
“It was enriching and gratifying to see others be as passionate and curious about the research that we had been working on throughout the summer. While we were working on our research, there was a lot of new equipment and procedures that we had to get used to working with, but the students adapted so easily and got the hang of the techniques pretty quickly.” Eliana
“I learned that it is not easy being on the other side of a lesson. Guiding students through a task you have done multiple times and completed successfully is a challenging but doable task; however, answering students’ questions is substantially harder. This is because the student’s perspective and strategies for formulating a question vary greatly per student. So, trying to understand their question and then rephrasing it to match their own reasoning was the toughest lesson. I was also surprised by the fact of how engaging students could be once they realize the opportunity they have been given, and this made the students really responsive to our guidance despite them being close to our age.” Taneeka
“As a graduate student who has helped with the first protocol used to start this program, it has been a truly rewarding experience to see how far-reaching this program has become. As a learning assistant, I was able to work with college students to help them understand research that I work with every day. As the saying goes, you don’t really understand your subject until you can explain it to someone else. Working with the students and the questions they asked helped me to think about my own research more deeply, and I hope I was able to help them understand the subject matter and serve as a support in their scientific and academic journeys as well.” Heather, graduate student in the Franco laboratory at UCLA.
Chemistry 2B student surveys: using oxDNA to simulate DNA nanostars
The Chemistry 2B students were given pre-surveys before the 5-week DNA nanotechnology curriculum and post-surveys afterward that asked questions about their experience, skills they developed, character, engagement, and applications to their careers. They indicated that they were very engaged by the nanotechnology curriculum, learned invaluable skills in DNA nanotechnology research, enjoyed working with graduate students and PCC nanostar researchers in a positive, supportive, and encouraging laboratory environment, and felt proud of themselves for accomplishing the goals set forth by the curriculum: forming and visualizing DNA condensates, simulating DNA nanostars using oxDNA, catalyzing colorimetric reactions using DNAzymes, localizing DNAzyme-catalyzed peroxidation to condensates, and bulk layering of DNA condensates. Most Chemistry 2B students enjoyed the oxDNA simulations; many said that it was challenging but rewarding. Students reported that modeling programs enhanced their understanding of the nanostructures and that it reinforced their concept of the nanoscale.
Chemistry 2B students’ quotes regarding the use of oxDNA in the synthetic biology lessons
- It allowed us to understand how the DNA strands connect together and visualize the DNA nanostars, understanding the structures of them.
- I liked it because it got me engaged; I thought of it as a video game where you can make something but still learn.
- It was definitely not easy, but it was cool seeing it through modeling programs. Loved it, it was intriguing!
- It was my first time working with the modeling programs, and it was very challenging, but Dr. JB provides very detailed steps that we can follow to work with the modeling programs, making it much easier.
- My favorite part of the new synthetic biology curriculum was modeling the DNAzyme using molecular visualization software. I thought it was really cool to be able to see these molecular structures up close on the computer and manipulate them. It helped reinforce my understanding of how DNA and enzymes interact at the atomic level.
- It was difficult at first, but through the help of professors and the student aids, I was able to have fun with the labs.
- It was amazing, and I am glad I got to do this for the first time.
- The making of our own models and putting the strands together was cool.
- It was a great experience to use a modeling program like oxDNA.
- Though there were parts where I was a little confused, I really had a great time.
- It was fun, cool, something different out of my comfort zone.
- I was very proud of creating my first nanostar on the computer. After waiting a long time for it to load, finally seeing it appear on the screen and being able to show it to my group was an amazing feeling!
- Even though we all worked on it together, seeing the final result made me feel really proud of what we accomplished.
- My experience is that, under the guidance of Dr. JB, I am more interested in modeling.
Themes that emerge from assessment surveys
By collecting information from students prior to the 5-week synthetic biology curriculum and post-surveys after the completion of the laboratory activities, it was possible to determine an overall attitude, identify key skills students developed, and determine whether they met the intended learning outcomes. In general, there was a very positive learning environment in the laboratory, and both students and learning assistants appreciated the supportive, welcoming environment. The Chemistry 2B students were very excited to participate in a novel curriculum centered on cutting-edge synthetic biology research, and in post assessment responses, there was an overall theme of gratitude. They appreciated the contextualization of chemistry concepts; they enjoyed the research, and they found the science to be exciting and accessible. Some students noted an initial difficulty with the oxDNA software, but most students thoroughly enjoyed using modern simulation tools to visualize the DNA nanostructures they formed in the lab. They were inspired by the passion and enthusiasm of the instructors and learning assistants for this field of science, and they reported a deeper understanding of scientific principles connected to these activities.
Chemistry 2B students at PCC had varied experiences with oxDNA simulations in their synthetic biology curriculum. Many found it challenging but rewarding, appreciating the detailed instructions and visual aids provided. Some students particularly enjoyed the modeling aspect, likening it to a video game that facilitated learning. They valued the opportunity to visualize DNA structures and interactions at the atomic level. However, a few students found the experience unpleasant and tedious. Despite initial difficulties, most students eventually became comfortable with the software and appreciated its educational value. The curriculum generally received positive feedback, with students expressing gratitude for the opportunity to engage with cutting-edge research and modern simulation tools. Learning assistants, including PCC/UCLA undergraduate and graduate students, reported feeling empowered by teaching their research to PCC students. They noted satisfaction in seeing students engage with the activities and appreciated the opportunity for career discussions. Overall, the synthetic biology curriculum created a positive learning environment, with students reporting a deeper understanding of scientific principles and an appreciation for the contextualization of chemistry concepts. The experience was seen as exciting and accessible despite some initial challenges with the software.
Key points from nanostar reflections regarding oxDNA in research include:
1. The tools helped bridge the gap between theoretical knowledge and practical applications in nanoscale research. 2. Visualization of DNA nanostars provided a deeper understanding of their structure and function, which was not possible through microscopy alone. 3. The experience highlighted the importance of modeling and simulation in scientific research and education. 4. Participants gained valuable skills in computational modeling and data interpretation. 5. The tools helped in understanding complex molecular dynamics and interactions. 6. Some participants faced initial challenges but found the learning process rewarding. 7. The experience inspired some to pursue further research in the field. 8. Many emphasized the potential of these tools for enhancing science education at various levels. Overall, the use of oxDNA and oxView significantly improved the participants’ comprehension of DNA nanostructures and their potential applications in biotechnology research.
Reflections regarding the experiences of the nanostars involved in DNA nanotechnology research and its associated teaching and outreach efforts emphasize that the project aimed to make research more accessible and inclusive for community college students and other underrepresented groups in STEM. Reina, Fabiana, and Vanessa, who were part of the research team, highlight the transformative nature of their experience. They emphasize how engaging with students as instructional assistants and participating in outreach workshops deepened their understanding of the research and strengthened their passion for science education. The use of oxDNA as a visualization tool was particularly effective in making the research more accessible and inspiring to students. The responses also include feedback from Learning Assistants (LAs) who helped teach DNA nanotechnology in Chemistry 2B at Pasadena City College. These LAs, including UCLA graduate students, shared their observations on student engagement, the challenges of teaching, and the mutual learning that occurred. They noted the students’ enthusiasm, curiosity, and ability to adapt to new techniques quickly.
Learning assistants (PCC/UCLA undergraduate students and UCLA graduate students)
The learning assistants, who were PCC / UCLA undergraduate students and UCLA graduate students, felt empowered as they taught their DNA nanotechnology research to the Chemistry 2B students at PCC. There was a general sense of satisfaction reported in seeing the PCC students engaged in these activities, which were developed based on our research. The PCC researchers communicated their enthusiasm for the science and oxDNA simulations, which was inspirational to the Chemistry 2B students, and the added benefit of career discussions and connections was appreciated by everyone involved.
Key points from the LAs’ experiences include: 1. The importance of providing moral support and validation to students 2. The positive, non-hierarchical learning environment that facilitated knowledge sharing 3. The excitement generated by hands-on experiences and seeing research results 4. The need to adapt teaching styles and use accessible language 5. The challenges of answering diverse student questions 6. The rewarding nature of inspiring and supporting students in their scientific journeys. Overall, the text highlights the positive impact of this research and education initiative on both the instructors and students involved, emphasizing the potential for such programs to foster equity and accessibility in scientific research.
Conclusions
This project was initiated by student researchers in Dr. JB’s laboratory, who had little to no knowledge of oxView and oxDNA and/or coding before being taught about the program in a graduate student-led seminar. After obtaining the basic knowledge of these programs and how to create and simulate a DNA nanostar from Mahdi Dizani, a graduate student in Dr. Franco’s laboratory at UCLA, the PCC nanostar researchers proceeded to replicate this process with all three nanostars (NS-15, NS-20, NS-25) with and without the sequence for a G-tetraplex (G4) extended from arm 4 and to attempt to fold the G4s used in their in vitro experiments. Using their burgeoning knowledge of the program, the student researchers then performed in silico experiments to fold the DNAzymes complexed with the nanostars. Various oxDNA parameters were tested to accomplish in silico folding of the G4 tetraplex, such as salt concentration and time (Fig. 9). Results indicate the folding of the G4 tetraplex appears to be unstable, and perhaps uncharacteristic of the true stability of the G-quadruplex structure [4,16]; however, these student-led simulations still provide a glimpse of what the quadruplex might look like in vitro, capturing the imaginations of the students and inspiring them in research. Further, the accessible visualization of student-designed nanostructure serves as an important visual learning tool in research and in courses. PCC researchers and Chemistry 2B students reported that the process of simulating DNA nanostars that they were working with in vitro (with and without G4s) has been of tremendous value in terms of visualizing the structures and learning about them. The students applied the insight gained through nanostar simulations to their research, which expands their knowledge from STEM courses at PCC and beyond: “It provides a platform for us to test our ideas and perform in silico experiments from the convenience of our own homes. Then, if we like, we can perform the same experiments (i.e. salt, temperature effects on nanostars) in the laboratory to see if our in silico results match the in vitro results.” Simulating DNA nanostructures in courses, research, and outreach had a positive impact on students and helped them visualize the structures they were imagining and building in the laboratory. Furthermore, by teaching and developing accessible tutorials, PCC researchers gained invaluable professional skills [23] that will prepare them for the STEM workforce [24].
Modifications to the oxDNA code may enable the true folding and visualization of the G4 tetraplex. Future experiments will be conducted to adjust the external force file in various ways to attempt to fold the DNAzyme into a more tetraplex-looking structure. Even though oxDNA is an invaluable tool for visualizing DNA nanostars and understanding their mechanical properties, the systematic simulations described above highlight an important limitation of the software in folding the G-quadruplex that should be noted by potential users.
Interestingly, during the in silico experiments with LA4 and 5xG3T using oxDNA, it was observed that LA4 seemed to fold more often and to a greater extent than 5xG3T (Figs. 7,8). A difference was also seen during in vitro experiments, where the DNAzyme-catalyzed peroxidation reaction using ABTS, a colorimetric substrate, often yielded a darker blue color when using LA4 compared to 5xG3T [25-29]. Given the limitations of oxDNA in folding a G4 tetraplex [17-19], it may be necessary to use experimental methods, such as cryo-electron microscopy (cryo-EM) or NMR solution structures, to gain structural information regarding the two DNAzymes to determine if there is truly a difference in folding. The folding of G-quadruplex structures (G4s) within the genome has been increasingly linked to critical biological processes and pathologies, including genomic instability and disease. Understanding the sensitivity of tetraplex folding in LA4 or 5xG3T-like DNA fragments could not only advance the design of nucleic acid-based biochemical reactors but also provide valuable insights into their biological relevance. By modeling the physical characteristics required for G4 folding and investigating mechanisms to induce unwinding, researchers can gain a deeper understanding of these structures and their potential biomedical applications [17, 28].
Accessible biomolecular simulations contribute to equity in STEM education. oxDNA simulations of DNA nanostructures have engaged a wide range of students, deepened their understanding of concepts learned in STEM courses, and inspired the imaginations of PCC nanostar undergraduate researchers. Simulations have informed authentic research projects in DNA nanotechnology and conveyed the power of coupling in silico work to in vitro studies. Regarding future use of oxDNA in nanotechnology research and teaching, it may be useful to attempt to model additional interactions, such as multiple nanostars or DNA strands used in toehold-mediated strand displacement (TMSD). TMSD was attempted on a DNA nanostar with a G4 tetraplex attached to arm four, and preliminary results suggest that TMSD prevented the DNAzyme-catalyzed peroxidation reaction [29]. Simulating interactions that occur in this process might help in designing an efficient and effective strategy of unfolding the G4 tetraplex, and thus ‘turning off’ the reaction. Further, computational models may help capture interactions between multiple nanostars with the same and/or different number of base pairs per arm to observe how they interact on different timescales [5].
By publishing the oxDNA simulation tutorial to YouTube [12], this research was made accessible to a wide audience; students simply need a laptop to design in silico experiments and carry out simulations using oxDNA. The accessibility of simulation programs used in DNA nanotechnology research, such as oxDNA, oxView, caDNAno, CanDo, and ENSnano, allows anyone to do this kind of research [30, 31]. It may even inspire the next generation of synthetic biology researchers to imagine and create their own nucleic acid-based structures with novel functions, and perhaps even enter their projects and designs into the BioMod competition for undergraduates [32].
Acknowledgments. We acknowledge NSF FMRG: Bio Award 2134772 for funding the PCC nanostars Future Manufacturing undergraduate research program. We thank our collaborators, Deborah Fygenson at UCSB and Paul Rothemund at Caltech, for their unwavering support and guidance. We would like to acknowledge the graduate students and postdocs in Elisa Franco’s laboratory at UCLA for their guidance, mentorship and support at the UCLA research techniques bootcamp. We are grateful for the enthusiastic participation of students in Dr. J. Blatti’s General, Organic, and Biochemistry courses at PCC in the Spring 2023 – 2025 semesters for their hard work and dedication as they participated in this novel synthetic biology curriculum, which included simulating DNA nanostars using oxDNA and oxView. Lastly, we are honored to serve as mentors and teachers to younger, underrepresented students in outreach, specifically at Girls Science Day 2024-2025 at PCC (Tech Savvy), as we make our synthetic biology research accessible to all.
Disclosures. The authors declare no conflicts of interest.
Supplemental Materials.
[1] D.Y. Zhang and E. Winfree, “Control of DNA strand displacement kinetics using toehold exchange,” J Am Chem Soc, vol. 131, no. 47, pp. 17303-17314, Nov. 2009, doi: 10.1021/ja906987s.
[2] S. Biffi et al., “Phase behavior and critical activated dynamics of limited-valence DNA nanostars,” PNAS, vol. 110, no. 39, pp. 15633-15637, Sep. 2013, doi: 10.1073/pnas.1304632110.
[3] Agarwal, D. Osmanovic, M. Dizani, M. A. Klocke, and E. Franco, “Dynamic control of DNA condensation,” Nat Commun, vol. 15, no. 1915, Mar. 2024, doi: 10.1038/s41467-024-46266-z.
[4] R. I. Adeoye et al., “Catalytic activities of multimeric G-quadruplex DNAzymes,”Catal., vol. 9, no. 7, p. 613, Jul. 2019, doi: https://doi.org/10.3390/catal9070613.
[5] A. Sengar, T.E. Ouldridge, O. Henrich, L. Rovigatti, and P. Šulc, “A primer on the oxDNA model of DNA: When to use it, how to simulate it and how to interpret the results,”Front. Mol. Biosci., vol. 8, no. 693710, Jun. 2021, doi: https://doi.org/10.3389/fmolb.2021.693710.
[6] E. Poppleton, R. Romero, A. Mallya, L. Rovigatti, P. Šulc, “OxDNA.org: a public webserver for coarse-grained simulations of DNA and RNA nanostructures,”Nucleic Acids Research, vol. 49(W1), W491–W498, 2021, doi: https://doi.org/10.1093/nar/gkab324.
[7] E. K. Snodin, F. Randisi, M. Mosayebi, P. Šulc, J. S. Schreck, F. Romano, T. E. Ouldridge, R. Tsukanov, E. Nir, A. A. Louis, J. P. K. Doye, “Introducing improved structural properties and salt dependence into a coarsegrained model of DNA,” J. Chem. Phys. vol. 142, no. 234901, 2015, doi: https://doi.org/10.1063/1.4921957.
[8] P.Šulc, F. Romano, T. E. Ouldridge, L. Rovigatti, J. P. K. Doye, A. A. Louis, “Sequencedependent thermodynamics of a coarsegrained DNA model,” J. Chem. Phys. vol. 137, no. 135101, 2012, doi: https://doi.org/10.1063/1.475413.
[9] L.Rovigatti, P. Šulc, I. Z. Reguly, F Romano, “A comparison between parallelization approaches in molecular dynamics simulations on GPUs,” J. Comput. Chem. vol. 36, no. 1, 1-8, 2015, doi: https://doi.org/10.1002/jcc.23763.
[10] T. E. Ouldridge, A. A. Louis & J. P. K. Doye, “Structural, mechanical, and thermodynamic properties of a coarse-grained DNA model,” Chem. Phys. vol. 134, no. 8, 085101, 2011, doi: https://doi.org/10.1063/1.3552946.
[11] S. Agarwal, D. Osmanovic, M.A. Klocke, & E. Franco, “The growth rate of DNA condensate droplets increases with the size of participating subunits,”ACS Nano, vol. 16, no. 8, pp. 11842-11851, Jul. 2022, doi: https://doi.org/10.1021/acsnano.2c00084
[12] Paredes, Fabiana. “Simulating DNA Nanostars Using oxDNA and oxView: Tutorial.” YouTube, 15 Aug. 2024, youtu.be/_FrL3UHvhdA.
[13] P.Travascio, Y. Li, & D. Sen, “DNA-enhanced peroxidase activity of a DNA-aptamer-hemin complex”, Chem. Biol. vol. 5, pp 505-517, 1998.
[14] T. Anand, R. Salman, B. Castañeda-Camacho, V. Gonzalez, E. Safar, E. Franco & J. L Blatti, “Investigating substrates Amplifu Red and ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) in the colorimetric detection of DNAzyme activity localized to DNA condensates,” J. Adv. Technol. Educ. vol. 4, no. 1, 2025, doi: https://doi.org/10.5281/zenodo.14933441.
[15] E. Terkazaryan & J.L. Blatti, “Dynamic GIF of 4 armed nanostars created on oxView and simulated using oxDNA.” YouTube, 9 June. 2025, https://youtu.be/G_R1Yf2wMC8.
[16] M. Bochman, K. Paeschke, & V. Zakian, “DNA secondary structures: stability and function of Gquadruplex structures,”Nat Rev Genet, vol. 13, pp. 770-780, Oct. 2012, doi: 10.1038/nrg3296.
[17] D. Karna et al., “Chemo-mechanical forces modulate the topology dynamics of mesoscale DNA assemblies,” Nat. Commun., vol. 14, no. 6459, Oct. 2023, doi: 10.1038/s41467-023-41604-z.
[18] A. Dutta et al., “Molecular states and spin crossover of hemin studied by DNA origami enabled single-molecule surface-enhanced Raman scattering,” Nanoscale, vol. 14, no. 44, pp. 16467-16478, 2022, doi: https://doi.org/10.1039/d2nr03664a.
[19] S. Haggenmueller, M. Matthies, M. Sample, & P. Šulc, “How we simulate DNA origami,” 2024, arXiv:2409.13206v1.
[20] N. M. Gravina, J. C. Gumbart, & H. D. Kim, “Coarse-Grained Simulations of DNA Reveal Angular Dependence of Sticky-End Binding,”J Phys Chem B, vol. 125, no. 16, pp. 4016-4024, Apr. 2021, doi: https://doi.org/10.1021/acs.jpcb.1c00432.
[21] L. Rovigatti. “External forces.” oxDNA. Accessed: Nov. 1, 2024. [Online.] Available: https://lorenzo-rovigatti.github.io/oxDNA/forces.html.
[22] N. Conrad, T. Kennedy, D. K. Fygenson, and O. A. Saleh, “Increasing valence pushes DNA nanostar networks to the isostatic point,” PNAS, vol. 116, no. 15, pp. 7238-7243, Mar. 2019, doi:https://doi.org/10.1073/pnas.1819683116.
[23] J.M. Ashcroft, J.L Blatti, V.J Jaramillo, Early Career Undergraduate Research as a Meaningful Academic Experience in which Students Develop Professional Workforce Skills: A Community College Perspective. In: Becoming a Chemist: Scaffolding Professional Skills into Undergraduate Curricula; Neiles, K.Y.; Mertz, P.S.; and Fair, J.D. Eds.; ACS Symposium Series, Vol. 1365; American Chemical Society: Washington DC, 2020; pp 281-299.
[24] J.L. Blatti, Invited Letter: Undergraduate research as a means to build a creative, resilient, and highly skilled biomanufacturing workforce. J. Adv. Tech. Educ. vol. 2, no. 2., 2023, doi: https://doi.org/10.5281/zenodo.8342997.
[25] J.Chen, Y. Zhang, M. Cheng, Y. Guo, J. Šponer, D. Monchaud, J.L. Mergny, H. Ju, & J. Zhou, “How Proximal Nucleobases Regulate the Catalytic Activity of G-Quadruplex/Hemin DNAzymes”, ACS Catalysis, vol. 8, no. 12, pp. 11352-11361, 2018.
[26] L. Stefan, H-J. Xu, C.P. Gros, F. Denat, & D. Monchaud, “Harnessing Nature’s Insights: Synthetic Small Molecules with Peroxidase-Mimicking DNAzyme Properties.” Chem. Eur. J., vol. 17, pp. 10857-10862, 2011, doi: https://doi.org/10.1002/chem.201101337.
[27] W. Li, Y. Li, Z. Liu, B. Lin, H. Yi, F. Xu, Z. Nie, S. Yao, “Insight into G-quadruplex-hemin DNAzyme/RNAzyme: adjacent adenine as the intramolecular species for remarkable enhancement of enzymatic activity”, Nucleic Acids Res. vol. 44, no. 15, pp. 7373-84, 2016.
[28] P. Lejault, J. Mitteaux, F.R. Sperti, & D. Monchaud, “How to untie G-quadruplex knots and why?” Cell Chem Biol, vol. 28, no. 4, pp. 436-455, Apr. 2021, doi: 10.1016/j.chembiol.2021.01.015.
[29] E. Terkazaryan, E. Safar, C. Khosravian, G. Mhlanga, M. Rivera, E. Franco, J.L. Blatti, “Exploring toehold-mediated strand displacement as a strategy to program DNAzyme-catalyzed peroxidation localized to DNA condensates,” J. Adv. Technol. Educ. vol. 5, no. 1, 2025, doi: https://doi.org/10.5281/zenodo.17215667.
[30] J. Garcia & J.L. Blatti, “Designing DNA origami with caDNAno: Tutorial on how to make a Square.” YouTube, 25 May. 2020, https://youtu.be/JnI1ilSP_tc.
[31] E. Terkazaryan & J.L Blatti, “oxDNA simulation of 4-arm DNA nanostars with G4 quadruplex on arm 4.” YouTube, 3 September. 2024, https://youtu.be/dWF95bJAnp0.
[32] BIOMOD Foundation. “BIOMOD Is an Annual Biomolecular Design Competition for Students.” BIOMOD, biomod.net/. Accessed 16 Jan. 2025.